One resonance structure of N2O is 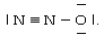 The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
Definitions:
Accounting Equation
The foundational principle of double-entry bookkeeping, stating that assets equal liabilities plus shareholders' equity.
Components
Elements or parts that combine to form a larger product or system.
Net Profit Margin Ratio
A profitability metric that shows the percentage of revenue that remains as net income after all expenses have been deducted.
Net Income
The total earnings of a company after accounting for all expenses and taxes, reflecting the company's profitability.
Q17: For the reaction: 3 Fe(s) + 4
Q21: A 40.2 L constant-volume cylinder containing 2.21
Q30: How much work, in joules, is done
Q33: A mixture of benzene and toluene has
Q35: For a reaction Rate = k[A][B]2, what
Q48: 100 mL of LiNO<sub>3</sub>, 0.241 M is
Q65: There are no types of crystalline solids
Q71: Activation energy is:<br>A) energy at the bottom
Q78: The ideal gas equation connects three basic
Q93: Which statement about the zero-point energy is