The average atomic mass of B is 10.80 u. Boron has only two stable forms 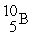 (10.00 u) and
(10.00 u) and 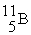 (11.00 u) . What is the natural percent abundance of
(11.00 u) . What is the natural percent abundance of 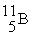 ?
?
Definitions:
Straight-Line Method
A depreciation technique where an asset's cost is evenly spread over its estimated useful life, resulting in equal annual depreciation expenses.
Partial-Year Depreciation
The calculation of depreciation for assets acquired or disposed of partway through the fiscal year, based on the portion of the year that the asset was in use.
Useful Life
The estimated period of time over which a tangible asset is expected to be useful for the purposes of the business.
Depreciable Cost
The original cost of a fixed asset minus its estimated salvage value, representing the amount subject to depreciation over the asset's useful life.
Q1: Convert 1400 mm to inches.<br>A) 5500 in<br>B)
Q8: One mole of H<sub>2</sub>SO<sub>4</sub> contains<br>A) two moles
Q14: In which of the following groupings are
Q55: The number of nonbonding electrons exceeds the
Q56: Acetylene gas may be produced by carefully
Q64: The simplest formula of a substance is
Q66: Two hundred mL of solution has a
Q75: The mutual attraction of gas molecules is
Q77: Which of the following is(are) isomer(s) of
Q87: Only metals in binary compounds use prefixes