The average atomic mass of B is 10.80 u. Boron has only two stable forms 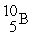 (10.00 u) and
(10.00 u) and 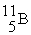 (11.00 u) . What is the natural percent abundance of
(11.00 u) . What is the natural percent abundance of 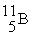 ?
?
Definitions:
Midlife
The period in an adult's life, often considered to range from approximately 40 to 60 years old, characterized by personal, social, and career reevaluations.
Grandparents
The parents of one's mother or father, who play a unique role in the family structure, often providing support, wisdom, and traditional values.
Havighurst's Vision
Robert Havighurst's theory that proposes people need to accomplish specific developmental tasks at various stages of life for successful aging.
Normalcy
The condition of being normal; the state of adhering to norms and standards perceived as typical.
Q10: Select the correct numerical value for the
Q27: Select the correct numerical value for the
Q32: For the set of ionic compounds, CaSO<sub>4</sub>,
Q45: Which of the following statements about activation
Q47: A 1.500 g sample of compound containing
Q49: An organic compound contains C, H, and
Q63: Two hundred thirty-seven pounds of ethanol with
Q66: Write the net ionic equation for the
Q70: Aspartame, C<sub>14</sub>H<sub>18</sub>N<sub>2</sub>O<sub>5</sub>, a low-calorie sweetener, is about
Q82: Which of the following is a chemical