According to Le Chatelier's principle, which of the following changes will shift the position of the equilibrium to the left for the reaction 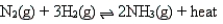
Definitions:
Work in Process Inventory
The account that tracks the costs associated with partially completed goods that are still in the process of being manufactured.
Unadjusted Cost of Goods Sold
Unadjusted Cost of Goods Sold (COGS) is the initial calculation of all costs directly associated with the production of goods before any adjustments for inventory changes or other factors.
Journal Entry
A record in the accounting journal that represents a business transaction and its effect on the accounts.
Manufacturing Overhead
All indirect costs associated with the production process, from maintenance expenses of the production facility to the supplies needed for operation.
Q18: 31.0 grams of the element phosphorus contain:<br>A)
Q29: For the reaction 2 Al + Fe<sub>2</sub>O<sub>3</sub>
Q41: For the electron configuration 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>5</sup>, choose the
Q44: Using the balanced equation for the combustion
Q47: Which of the following substances has a
Q49: If two solutions with concentrations of 0.4
Q54: The result of addition can have more
Q62: For the following intermolecular force descriptios, select
Q66: Avogadro's number of sulfur (S) atoms would
Q96: Which of the following represents a neutralization