According to Le Chatelier's principle, which of the following changes will shift the position of the equilibrium to the left for the reaction 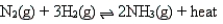
Definitions:
Fair Value
An estimation of the market value of an asset or liability, reflecting the amount for which it could be exchanged or settled between knowledgeable, willing parties.
Reporting Unit
A component of an organization for which discrete financial information is available and is reviewed regularly by the operations’ segment manager.
SEC
Stands for the Securities and Exchange Commission, which is a U.S. federal agency responsible for enforcing federal securities laws and regulating the securities industry, including stock and options exchanges.
Q7: Whether a reaction is exothermic or endothermic
Q7: The correct form of the equation for
Q23: Consider the mathematical expressions for gas laws
Q29: Assuming that the proposed reactants are mixed
Q29: CO<sub>2</sub> and H<sub>2</sub> are allowed to react
Q32: Choose the correct number of shared electrons
Q43: In the redox reaction 2MnBr<sub>3</sub> + SnBr<sub>2</sub>
Q56: Characterize EACH of the following statements as
Q68: Which one of the following conversion factors
Q74: Which of the following would have the