Multiple Choice
What is the molarity of an HNO3 solution if 24.1 mL of a 0.250 M Ba(OH) 2 solution are needed to titrate a 15.0 mL sample of the acid according to the equation below? Ba(OH) 2(aq) + 2 HNO3(aq) 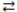 2 H2O(l) + Ba(NO3) 2(aq)
2 H2O(l) + Ba(NO3) 2(aq)
Definitions:
Related Questions
Q17: According to the authors,what are the advantages
Q18: A group in the middle stage has
Q19: The numerical value of the universal gas
Q20: One member shared some deep and personal
Q24: During the first session of a group
Q25: A strong acid readily donates a proton,
Q26: Leaderless support groups,leader-led support groups,and therapy groups
Q32: It seems apparent that the future for
Q35: Many counseling,therapy,and growth groups go through a
Q86: In a chemical reaction, the actual yield