When phosphoric acid (H3PO4) dissolves in water, the equilibrium shown below is established. If Ka = 7.5 × 10-3 for H3PO4, which statement concerning an aqueous solution of phosphoric acid is correct?
H3PO4 + H2O 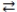 H3O+ + H2PO4-
H3O+ + H2PO4-
Definitions:
Compensating Differences
Differences in the wages received by workers in different jobs to compensate for the nonmonetary differences between the jobs.
Wage Rates
Wage rates are the standard amount of compensation given to employees for their labor, typically expressed per hour or day.
Job Characteristics
Attributes or aspects of a job that influence workers' motivation and satisfaction.
Wage Differentials
The variations in wage rates due to factors such as occupation, experience, education, industry, location, and economic conditions.
Q5: If cells are placed in a hypertonic
Q5: Polypropylene, a typical plastic, is an example
Q7: It is important that the group leader
Q22: The type(s)of intermolecular forces exhibited by methane
Q28: A group of first-generation college students meet
Q38: Members who will miss the closeness of
Q39: This leader finished her Master's degree last
Q43: How can making a topic outline be
Q51: Sarin, a nerve gas once used as
Q51: According to the authors,a leader can use