Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 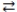 COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
Definitions:
Cost Curves
Graphical representations that show the cost of production at different levels of output.
Identical
Identical means exactly the same, without any difference in form, nature, or substance, often used to describe objects, products, or entities.
Long-run Equilibrium
A state in which all factors of production and inputs in a market are fully adjusted to the market conditions, allowing for steady-state operation without excess supply or demand.
Perfectly Competitive
A market structure characterized by a large number of small firms, homogeneous products, and no barriers to entry or exit.
Q8: A particular wine contains 11.2% (v/v)ethanol. What
Q13: Which of the following IS a good
Q38: Which food product does not rely on
Q45: What are the benefits of using dyads
Q49: The addition of an acid to water
Q49: Which bond is the strongest?<br>A)H-Br<br>B)H-Cl<br>C)H-F<br>D)H-I
Q56: What is the ion symbol for an
Q61: Step 7 of the citric acid cycle
Q68: What is the name of the SO<sub>4</sub><sup>2-</sup>
Q83: Which ion is the strongest base?<br>A)Br<sup>-</sup><br>B)F<sup>-</sup><br>C)I<sup>-</sup><br>D)NO<sub>3</sub><sup>-</sup>