Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 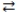 COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
Definitions:
Intangible Asset
A non-physical asset having value due to its intellectual or proprietary characteristics, such as patents, trademarks, and goodwill.
Amortized
The gradual reduction of a debt through regular payments that cover both interest and principal over time.
Finite Life
The limited time span during which an asset is expected to be useful in the operations of a business, after which it becomes obsolete or non-functional.
Amortized
This refers to the process of gradually paying off a debt through regular payments over a set period, factoring in both principal and interest.
Q14: The conversion of Fe<sup>3+</sup> to Fe<sup>2+</sup> in
Q17: Aspartic acid is an amino acid used
Q33: An iron (III)ion has 29 protons and
Q36: Although all of the following are important,the
Q37: The reaction: Mg(s)+ 2 HBr(aq) <span
Q39: Since living cells are surrounded by biological
Q44: How much ATP results from the transformation
Q77: Six of the steps of gluconeogenesis use
Q78: Which of the following statements concerning a
Q83: Which ion is the strongest base?<br>A)Br<sup>-</sup><br>B)F<sup>-</sup><br>C)I<sup>-</sup><br>D)NO<sub>3</sub><sup>-</sup>