Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 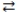 COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
Definitions:
In-Group/Out-Group
A social categorization that distinguishes between members (in-group) who belong to a specific group and non-members (out-group) who do not.
Prejudice
Is an attitude that judges a person on his or her group’s real or imagined characteristics.
Emergencies
Situations that arise suddenly and require immediate action due to posing a threat to health, life, property, or environment.
Groupthink
Is group pressure to conform despite individual misgivings.
Q9: All of the following represent common mistakes
Q22: If a synthesis has four steps and
Q38: It is important to ensure that all
Q42: When introducing an exercise in a group
Q43: Potassium metal (K)reacts violently when added
Q46: Which of the following molecule(s)is(are)polar?<br>A)CO<sub>2</sub><br>B)CH<sub>4</sub><br>C)CBr<sub>4</sub><br>D)CHBr<sub>3</sub><br>E)More than one
Q50: The solubility of a substance is best
Q53: The larger the K for a reaction,
Q55: Which sequence is not possible?<br>A)GAGAA<br>B)TAGGG<br>C)UTAGA<br>D)CCCCC
Q58: Ammonia (NH<sub>3</sub>)is synthesized by the reaction of