Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 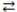 COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2.
COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2.
Definitions:
Yield Change
A change in the rate of return on an investment, often used in the context of bonds to indicate a change in interest rates or bond prices.
Price Decline
A decrease in the market price of an asset or security over a certain period.
Zero-Coupon Bond
A bond that does not pay periodic interest payments and is issued at a discount to its face value, which is paid at maturity.
Annual Coupon Bond
A type of bond that pays interest to the bondholder on an annual basis.
Q4: The cells in heart tissue have more
Q5: The actual yield of a product in
Q10: What is the abbreviation of the structure
Q29: _ is the main source of energy
Q29: What are the bond angles in a
Q36: When the leader directs the behaviors,discussion,or attention
Q45: Which structure represents a nucleotide?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5866/.jpg"
Q53: How many molecules of coenzyme A are
Q70: What is the pH of a buffer
Q89: How many hydrogen bonds form between a