The reversible reaction: PCl3(g) + Cl2(g)  PCl5(g) , has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions?
PCl5(g) , has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions? 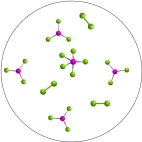
Definitions:
Homogamy
The practice or tendency of people to marry others who share similar cultural, social, or economic backgrounds.
Monogamy
The practice of marrying (or being in a relationship with) one person at a time.
Cohabitation
Living together as a romantic couple without being married.
Domestic Work
Tasks and activities performed to manage and maintain a household, such as cooking, cleaning, and childcare.
Q2: Which element has a variable charge?<br>A)Na<br>B)Fe<br>C)Al<br>D)C
Q16: A peanut butter and jelly sandwich contains
Q23: The complete catabolism of glucose forms _
Q26: Discuss why using an exercise in the
Q32: A solution can be made less concentrated
Q47: The rate of a chemical reaction increases
Q55: The law of conservation of energy states
Q57: A main group element is especially stable
Q66: What is the maximum volume of a
Q67: Cortisol is an anti-inflammatory agent that also