Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 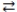 COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel, the equilibrium will shift to the right.
Definitions:
PTSD
Post-Traumatic Stress Disorder, a mental health condition triggered by experiencing or witnessing a terrifying event, characterized by flashbacks, nightmares, and severe anxiety.
Reexperiencing
The involuntary reliving of traumatic events, often a symptom of PTSD.
Wounds Dehisced
A surgical complication where a wound ruptures along a surgical incision, often due to improper healing or infection, leading to the separation of the previously joined edges.
Reconstructive Surgery
A surgical procedure aimed at restoring the appearance or function of a body part.
Q2: The solubility of KI in water at
Q3: The correct name for Ag<sub>2</sub>O is _.<br>A)silver
Q3: A glucogenic amino acid may also be
Q4: Asking members to position themselves somewhere between
Q17: When a sample of gas is compressed
Q17: Adlerian psychology emphasizes that all behavior is
Q25: A strong acid readily donates a proton,
Q31: In bonding, main group elements gain, lose,
Q77: Six of the steps of gluconeogenesis use
Q86: When the equilibrium constant for a reversible