Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 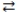 COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
Definitions:
Job Security
The probability that an individual will keep their job; a secure job has a low risk of being lost.
Employee Attitudes
The beliefs and feelings employees have about their job, role, and organization, which can significantly affect their job performance and satisfaction.
Human Capital Component
Refers to the economic value of an employee's skill set, including education, training, and experience.
Q5: Members lightly put their hands on each
Q16: Under no circumstances is it appropriate for
Q36: One advantage of a theory like Rational
Q38: It is important to ensure that all
Q38: The use of which of the following
Q51: The phosphorylation of glucose provides enough energy
Q57: What is the molecular shape around the
Q59: Which is a molecular solid?<br>A)MgCl<sub>2</sub><br>B)Au<br>C)SiO<sub>2</sub><br>D)graphite<br>E)sucrose (C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>)
Q80: When <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q86: Under some circumstances, when glucose is unavailable