Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 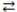 COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2.
COCl2(g), where H = -108.6 kJ. When the temperature of the reaction vessel decreases, the system responds by forming more COCl2.
Definitions:
Tight Spaces
Areas or locations with a limited or confined amount of space, posing challenges for movement or fitting in equipment.
Dimension Lines
Lines on a drawing that represent the measurement of a feature, accompanied by arrows and numerical values to denote length.
Object Lines
Thick solid lines in technical drawings that represent the visible edges of an object.
Two Millimeters
A length measurement equivalent to 0.07874 inches, often used to specify the dimension of small features in engineering and technical drawings.
Q5: What is the balanced equation for the
Q13: Which of the following is a
Q23: Walking at a brisk pace burns off
Q28: Because groups involve sharing,the amount of _
Q29: In a two-step synthesis where the first
Q37: The reaction: Mg(s)+ 2 HBr(aq) <span
Q48: Aluminum metal and oxygen gas are obtained
Q79: A 200.-mg ibuprofen (C<sub>13</sub>H<sub>18</sub>O<sub>2</sub>)tablet contains greater than
Q82: The correct name for CaHPO<sub>4</sub> is calcium
Q83: A polynucleotide contains _<br>A)one free phosphate group