The combustion of methanol takes place according to the reaction 2CH3OH(l) + 3O2(g) 2CO2(g) + 4H2O(l)
Calculate H for the combustion of 1 mol of methanol under standard conditions. Use the following standard enthalpies of formation: 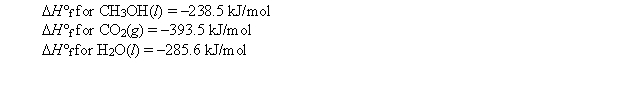
Definitions:
DFL
Degree of Financial Leverage; a measure that shows how much a company's earnings per share (EPS) are affected by changes in its operating income, indicating the volatility of earnings.
Dividends Per Share
The amount of dividends paid to shareholders, divided by the number of outstanding shares of a company's stock.
Stock Price
The present rate at which a company's stock is bought and sold on the stock exchange.
Present Value
The present worth of a future amount of money or sequence of cash payments, calculated using a particular return rate.
Q10: The enthalpy of fusion of ice is
Q10: Ashlyn was asked by her research advisor
Q14: Why might a researcher choose to use
Q25: What volume of 0.504 M barium nitrate
Q53: The file drawer problem is:<br>A) when researchers
Q54: After 300.0 mL of 0.200 M NaOH
Q78: Which of the following includes distress,concern,and lowered
Q89: Nelson is applying for a job within
Q102: Rules like "Participants have the right to
Q132: For ammonia, K<sub>b</sub> is 1.8 <font face="symbol"></font>