You have a solution of 0.10 M Cl- and 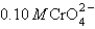 . You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
. You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
Definitions:
Responding
The act of replying or reacting to a message, situation, or stimulus.
Email Signature
A block of text automatically appended at the bottom of an email message containing the sender's contact information and sometimes, a legal disclaimer.
Personalization
The process of tailoring content, recommendations, or experiences to individual users or consumers based on their preferences or behavior.
Subject Field
A specific area of knowledge or study.
Q9: Three 1.00-L flasks at 25°C and 725
Q17: The difference between nonempirical and empirical research
Q26: Specific heat capacities are tabulated on a<br>A)
Q31: Calculate the equilibrium concentration of N<sub>2</sub>.<br>A) 1.5
Q47: A balloon contains an anesthetic mixture of
Q55: The volume of a balloon is 2.74
Q83: When the equation Cl<sub>2</sub> <font face="symbol"></font> Cl<sup>-</sup>
Q91: First-born children may think they are braver
Q97: Into a 3.90-liter container at 23°C are
Q113: Differentiate between a formation constant and a