Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 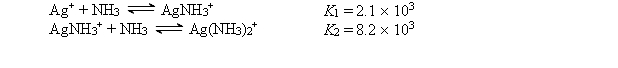 The concentration of Ag(NH3) 2+ at equilibrium is
The concentration of Ag(NH3) 2+ at equilibrium is
Definitions:
Preparing Environment
The process of arranging or modifying a setting to make it suitable for specific tasks or to enhance its functionality.
Physical Setting
The natural or built environmental context in which events occur, influencing the behavior and interactions of its occupants.
Financial Support
Monetary assistance provided to individuals, organizations, or governments to help them meet their financial needs or goals.
Closed Membership
Closed Membership implies a restricted entry system where members are admitted based on certain criteria, and new entries are limited or require special admission processes.
Q1: Empirical research is:<br>A) using one's own thoughts
Q4: If solid sodium cyanide (NaCN) is dissolved
Q15: _ is the process of reflecting on
Q31: NaHCO<sub>3</sub> is the active ingredient in baking
Q46: Consider the following reaction: 2SO<sub>2</sub>(g) + O<sub>2</sub>(g)
Q46: One benefit of attending a research conference
Q63: For the compound MX, K<sub>sp</sub> is 2.00
Q80: Psychologists replicate previous findings for each of
Q88: Consider the reaction between 50.0 mL of
Q163: A 10-mL sample of tartaric acid is