A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN) 32-: 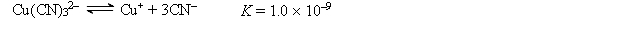 Calculate the solubility of CuBr(s) (Ksp = 1.0 10-5) in 1.0 L of 1.0 M NaCN.
Calculate the solubility of CuBr(s) (Ksp = 1.0 10-5) in 1.0 L of 1.0 M NaCN.
Definitions:
Managed Float System
An exchange rate system that combines features of freely floating rates with sporadic intervention by central banks.
Bretton Woods Agreement
A 1944 agreement that established fixed foreign exchange rates for major currencies, as well as the IMF and the World Bank.
Gold Standard
A monetary arrangement where the valuation of a country's currency or paper bills is pegged directly to gold.
Bretton Woods System
was a monetary order established in 1944, which set up fixed exchange rates, the International Monetary Fund (IMF), and the World Bank to regulate international financial and monetary affairs.
Q9: Calculate w<sub>AC</sub>.<br>A) 0<br>B) 30 L•atm<br>C) -30 L•atm<br>D)
Q10: (Scenario II)Alissa just found out her boyfriend,Jake,cheated
Q16: Given the equation S(s) + O<sub>2</sub>(g) <font
Q41: Derive the Henderson-Hasselbalch equation from the K<sub>a</sub>
Q58: The volume of a helium balloon is
Q69: Consider the following reaction: CH<sub>4</sub>(g) + 4Cl<sub>2</sub>(g)
Q95: Calculate the pH of a solution made
Q99: A 0.050 M aqueous solution of a
Q128: What combination of substances will give a
Q139: You have two salts, AgX and AgY,