The cation M2+ reacts with NH3 to form a series of complex ions as follows: 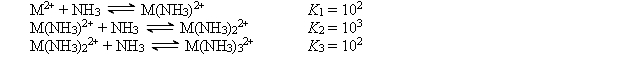 Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Definitions:
Lead Second
The position or role of supporting leadership by providing feedback, direction, and assistance to the primary leader or team.
Ethical Leadership
The practice of leading by demonstrating ethical behavior, and making decisions that reflect fairness, honesty, and respect for others.
Immediate Supervisor
The person who has direct authority over an employee and is responsible for managing their performance and day-to-day activities.
CEO
The Chief Executive Officer is the top executive in a company or organization, bearing the ultimate responsibility for decision-making.
Q14: Raquel is interested in determining whether astrological
Q27: For a particular process q = -10
Q32: Determine the number of molecules of acetylsalicylic
Q35: State Le Châtelier's principle.
Q52: What volume of 0.25 M HNO<sub>3</sub> is
Q64: Mixing 40.0 mL of a 4.00 M
Q79: Calculate the concentration of chromate ion, CrO<sub>4</sub><sup>2</sup><sup>-</sup>,
Q85: Consider a 100.0-mL sample of a 0.10
Q98: Otto is examining whether paying for purchases
Q98: A 3.54-g sample of lead(II) nitrate, Pb(NO<sub>3</sub>)<sub>2</sub>,