The Ag+ ion reacts with NH3 to form the following complex ions: 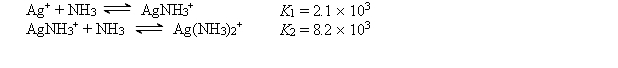 AgCl (Ksp = 1.6 *10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
AgCl (Ksp = 1.6 *10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
Definitions:
Truth Table
A table used in logic to determine the truthfulness of various combinations of propositions.
Atomic Sentences
The simplest kind of sentences in logic that cannot be broken down into smaller parts, representing basic facts or assertions.
Truth Values
The attribution of truthfulness to a statement, typically classified as either true or false in classical logic.
Truth Table
A mathematical table used in logic to determine the validity of an argument or to show the truth value of a logical expression based on all possible scenarios.
Q10: The primary avenue by which psychologists share
Q18: Consider a solution of 2.0 M HCN
Q22: _ means to base claims on scientific
Q26: Mg + HCl <font face="symbol"></font> MgCl<sub>2</sub> +
Q45: When a mixture is prepared from 15.0
Q56: What is the pH at point III?<br>A)
Q63: How much water must be added to
Q90: Consider five solutions that all have the
Q110: Which of the following titration curves schematically
Q111: The observed solubility of the salt MX