You have a solution of 0.10 M Cl- and 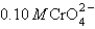 . You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
. You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
Definitions:
Ounce
A unit of weight used primarily in the United States, Canada, and the United Kingdom, equal to one-sixteenth of a pound or about 28.35 grams.
Futures Contracts
Futures contracts are standardized legal agreements to buy or sell a particular commodity or financial instrument at a predetermined price at a specified time in the future.
Payoff
The potential gain or loss that will result from a specific investment or decision.
Ounce
A unit of weight commonly used in the United States and the United Kingdom, equal to one-sixteenth of a pound or approximately 28.35 grams.
Q22: Consider two samples of helium in separate
Q26: Specific heat capacities are tabulated on a<br>A)
Q33: Consider the equation 2A(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg" alt="Consider
Q40: Two metals of equal mass with different
Q46: Consider the following reaction: 2SO<sub>2</sub>(g) + O<sub>2</sub>(g)
Q51: The difference between basic and applied research
Q56: _ is a bias in which people
Q70: When 0.72 g of a liquid is
Q74: Calculate the percentage of pyridine (C<sub>5</sub>H<sub>5</sub>N) that
Q92: Operational definitions are a critical part of:<br>A)