Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 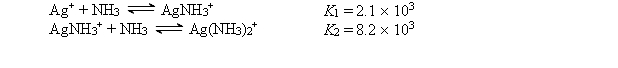 The concentration of Ag(NH3) 2+ at equilibrium is
The concentration of Ag(NH3) 2+ at equilibrium is
Definitions:
Mutual Funds
Mutual funds are investment vehicles that pool money from numerous investors to purchase a diversified portfolio of stocks, bonds, or other securities.
U.S. Treasury Bonds
Long-term debt securities issued by the United States Department of the Treasury with a maturity of more than ten years.
Equity Mutual Fund
A corporation that pools the funds of investors, including small investors, and uses them to purchase a bundle of stocks.
U.S. Households
Living arrangements in the United States that include all the people who occupy a housing unit, such as families, roommates, or individuals living alone.
Q4: Iron is produced from its ore by
Q14: Find the percent oxygen (atoms) by mass
Q20: The Moneyball approach to baseball represents a
Q24: Titrating 30.00 mL of a saturated calcium
Q28: Consider two samples of gas at the
Q38: Consider the equation 2A(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg" alt="Consider
Q54: How many atoms of hydrogen are present
Q66: If K<sub>a</sub> for HCN is 6.2 <font
Q77: An alkali metal oxide contains 46.45% metal
Q83: A sample of iron weighing 18.4 g