A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN) 32-: 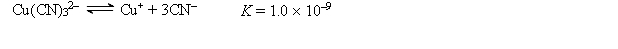 What is the concentration of CN- at equilibrium?
What is the concentration of CN- at equilibrium?
Definitions:
Operating Activities
Activities related directly to the production, sale, and delivery of a company's products and services, as reflected in its cash flow.
Cash Collected
The total amount of money received by a company from its various sources during a particular period, including sales, investments, and financing activities.
Cash Equivalents
Short-term investments with original maturities of three months or less that are readily convertible to cash and whose value is unlikely to change.
Q9: (Scenario III)After hearing about this study Kevin
Q27: Between-subjects design is to within-subject design as:<br>A)
Q32: Calculate the density of nitrogen at STP.<br>A)
Q35: Generally speaking,_ research serves as the foundation
Q40: Two metals of equal mass with different
Q47: You have solutions of 0.200 M HNO<sub>2</sub>
Q50: A 0.240 M solution of the salt
Q54: Calculate the pH of a solution made
Q92: Operational definitions are a critical part of:<br>A)
Q98: A 50.00-mL sample of 0.100 M KOH