Given the following values of equilibrium constants: 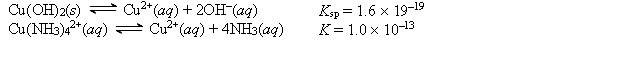 What is the value of the equilibrium constant for the following reaction? Cu(OH) 2(s) + 4NH3(aq)
What is the value of the equilibrium constant for the following reaction? Cu(OH) 2(s) + 4NH3(aq)  Cu(NH3) 42+(aq) + 2OH-(aq)
Cu(NH3) 42+(aq) + 2OH-(aq)
Definitions:
Selling Price
The sum of money required for a product or service, which directly affects the income produced through sales.
Fixed Manufacturing Overhead
Fixed costs related to manufacturing that do not change with the level of production, such as factory rent and salaries of permanent staff.
Direct Labor
The wages and other compensation paid to employees who are directly involved in the production of goods and services.
Special Order
A customer request for a product or service that is not normally offered by the business, often requiring unique production or procurement efforts.
Q3: Calculate the pH of a 2.0 <font
Q12: For a certain reaction at 25.0°C, the
Q21: To calculate the concentration in molarity of
Q48: Consider the following numbered processes: <br>1. A
Q51: How many moles of HCl(g) must be
Q62: For the reaction AgI(s) + (1/2)Br<sub>2</sub>(g) <font
Q67: For which gas are the collisions elastic?<br>A)
Q70: If 5.0 kJ of energy is added
Q93: The salt BX, when dissolved in water,
Q161: 400.0 mL of 1.00 M NaOH<br>A) 12.42<br>B)