Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 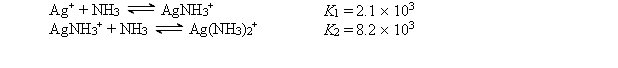 The concentration of Ag+ at equilibrium is
The concentration of Ag+ at equilibrium is
Definitions:
Second-order Conditioning
A process in which a neutral stimulus becomes a conditioned stimulus by being paired with a previously conditioned stimulus.
Eating Behavior
The range of psychological and physiological processes that influence how and what individuals consume for food.
Stimulus Discrimination
The learned ability to differentiate between similar stimuli and respond only to the original stimulus in conditioning.
Extinction
In behavior psychology, the gradual weakening and eventual disappearance of a conditioned response when the conditioned stimulus is no longer paired with the unconditioned stimulus.
Q13: In a nonexperimental design the _ is
Q13: Calculate the equilibrium concentration of NH<sub>3</sub>(g).<br>A) 3.7
Q35: Generally speaking,_ research serves as the foundation
Q36: Consider three 1.0-L flasks at STP. Flask
Q48: For the reaction of sodium bromide with
Q57: A 2.58-g sample of NaOH(s) is added
Q62: An aqueous solution of barium nitrate reacts
Q63: At a given temperature, the equilibrium constant
Q95: With regard to characteristics of a good
Q134: After 350.0 mL of 0.200 M NaOH