The cation M2+ reacts with NH3 to form a series of complex ions as follows: 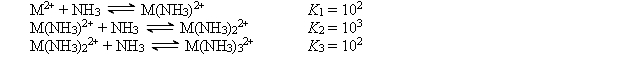 Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Consider an experiment in which 1.0 *10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Definitions:
Interest Rate
The amount charged, expressed as a percentage of principal, by a lender to a borrower for the use of assets.
Net Present Value
The difference between the present value of cash inflows and the present value of cash outflows over a period of time.
Interest Rate
The proportion of a loan that is charged as interest to the borrower, typically expressed as an annual percentage of the outstanding loan.
Discounting
The procedure used to calculate the present value of future income, which is inversely related to both the interest rate and the amount of time that passes before the funds are received.
Q4: Mark wonders whether he should pursue a
Q5: Nitric oxide, an important pollutant in air,
Q9: A 59.00-mL sample of 0.0650 M HCN
Q34: A solution contains 0.34 M HA (K<sub>a</sub>
Q37: Which of the following is an ethical
Q41: Keeping researchers and participants "blinded" to (or
Q62: Force per impact versus the molar mass
Q70: Which titration curve would result from the
Q152: The two salts AgX and AgY have
Q170: The concentration of Al<sup>3+</sup> in a saturated