The Ag+ ion reacts with NH3 to form the following complex ions: 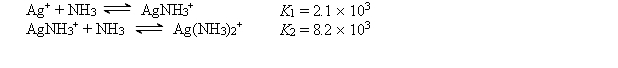 AgCl (Ksp = 1.6 *10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
AgCl (Ksp = 1.6 *10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
Definitions:
Present Obligation
A duty or responsibility to act or perform in a certain way.
Goodwill
The intangible asset that arises when a buyer acquires an existing business but pays more than the fair market value of the net assets.
AASB 3
is the Australian Accounting Standards Board's standard on Business Combinations, detailing the accounting treatment for merging businesses, including the recognition and measurement of assets, liabilities, and any non-controlling interest.
IFRS 3
The International Financial Reporting Standard that deals with the accounting for business combinations, requiring assets, liabilities, non-controlling interest, and goodwill to be accounted for at fair value.
Q12: Define an outlier and give an example
Q12: Cr<sub>2</sub>O<sub>7</sub><sup>2</sup><sup>-</sup> + I<sup>-</sup> <span class="ql-formula"
Q28: Chromate ion is added to a saturated
Q33: Dr.Pelham cautions her students against merely reviewing
Q36: Calculate the pH of a 0.30 M
Q44: 21.4 g of Al and 14.6 g
Q46: The molar mass of the insecticide dibromoethane
Q82: A 135.0-mL sample of a 0.25 M
Q83: You dissolve 1.24 g of an unknown
Q84: Which of the following is a conjugate