Based on the van der Waals equation of state 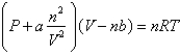 Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas. 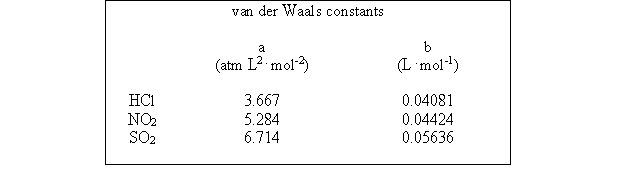
Definitions:
Seismographs
Seismographs are instruments that measure and record the motion of the ground, including those of seismic waves generated by earthquakes, volcanic eruptions, and other seismic sources.
Plate Divergence
A tectonic process involving the moving apart, or divergence, of two tectonic plates.
Oceanic Trench
Deep depressions in the ocean floor caused by the subduction of one tectonic plate under another.
Magmatic Belt
A region characterized by extensive igneous rock formation, often associated with plate boundaries and volcanic activity.
Q10: At equilibrium, the partial pressure of HCl
Q42: The complex ions of Zn<sup>2+</sup> are all
Q50: Which of the following is optically active
Q51: A student weighs out 0.568 g of
Q58: The molar mass of the insecticide dibromoethane
Q74: Consider the following unbalanced equation: KO<sub>2</sub> +
Q75: A mixture of solid NaCl and CaCl<sub>2</sub>
Q90: Consider the following two reactions. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg"
Q102: The pH of a solution made of
Q151: Silver acetate (AgC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>) is a sparingly soluble