Based on the van der Waals equation of state 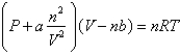 Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas. 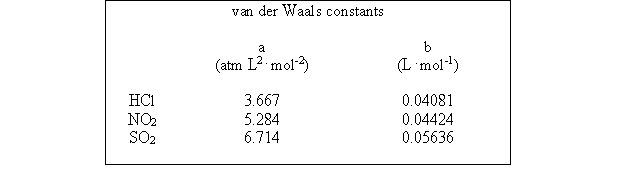
Definitions:
Population Age Structure
The distribution of individuals in a population according to their ages, which influences growth rates and demographic trends.
Prereproductive
Referring to organisms or life stages that have not yet reached the age or stage where they can reproduce.
Demographic Transition Model
A theoretical model that describes the stages a country goes through as it transitions from a non-industrial to an industrial economic system, influencing birth and death rates and overall population growth.
Q15: Calculate the following ratios for a gas
Q27: Which gas, He or H<sub>2</sub>O vapor, has
Q51: The half-life for electron capture for <img
Q57: For which of the following reaction(s) is
Q69: Consider the following reaction:<br>2Al(s) + 3Cl<sub>2</sub>(g)
Q77: An indicator HIn has K<sub>a</sub> = 1
Q82: A 135.0-mL sample of a 0.25 M
Q87: The conjugate acid and conjugate base of
Q108: A gaseous mixture containing 1.5 mol Ar
Q109: Isomers have<br>A) different molecular formulas and different