Given below are the temperatures at which two different liquid compounds with the same empirical formula have a vapor pressure of 400 torr. 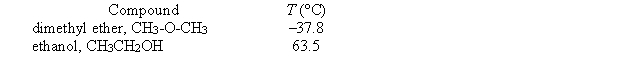 Which of the following statements is false?
Which of the following statements is false?
Definitions:
Vasodilation
The widening of blood vessels, which decreases blood pressure and allows increased blood flow.
Active Natural Immunity
Immunity that develops after exposure to a disease-causing organism, leading to the production of antibodies and immunological memory.
Developing Disease
The process by which a disease begins to form or increase in severity, involving the progression from initial symptoms to more severe conditions.
Vaccinated
Being vaccinated refers to having received a vaccine, which is a substance that stimulates the body's immune response against diseases.
Q3: Which is the most reactive form of
Q7: Which of the following processes increases the
Q15: When the U-235 nucleus is struck with
Q19: How many of the following exhibit resonance?
Q25: Which of the following complexes would be
Q33: The nuclide <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg" alt="The nuclide
Q53: Determine the amount of energy needed to
Q76: The balanced equation for the reaction of
Q95: A first-order reaction is 54% complete at
Q108: For which of the following elements does