A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C, and the pressure is increased to 8 atm. Based on the phase diagram below, what will happen? 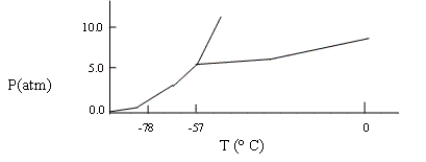
Definitions:
Yield To Maturity
the total return anticipated on a bond if the bond is held until it matures, considering both interest payments and capital gain.
10 ½% Bond
A bond that pays an annual interest rate of ten and a half percent of its face value.
Yield To Maturity
The total return anticipated on a bond if it is held until its maturity date.
8 ½% Bond
A bond that pays an annual interest rate of 8.5% on its face value.
Q12: Atoms that are sp<sup>3</sup> hybridized form _
Q22: Select the best Lewis structure for acetone,
Q27: nitric acid
Q39: How many protons, neutrons, and electrons does
Q41: Choose the most likely pattern for the
Q47: What is the correct formula for barium
Q48: A solution of two liquids, A and
Q54: A sample of wood from an Egyptian
Q60: How many neutrons are emitted?<br>A) 5<br>B) 6<br>C)
Q106: The triple point of iodine is at