Based on the phase diagram shown below, which of the following statements are correct?
I. Sublimation occurs at a point in the transformation that falls along a straight line from point A to point F.
II. C and E represent points where the gas and liquid phases are in equilibrium.
III. (Hvap can be measured at point B) .
IV. Molecules at point D have a greater average kinetic energy than those at point F.
V. The temperature at point E is called the critical temperature of the compound. 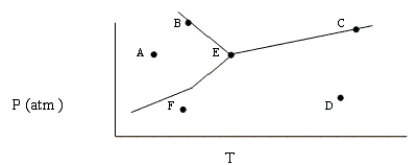
Definitions:
Attitude
A fairly stable evaluative tendency to respond consistently to some specific object, situation, person, or category of people.
Behaviour
The actions, reactions, or conduct of an individual or system in response to external or internal stimuli.
Direct Experience
Knowledge or skill acquisition that comes from personally engaging in a task or activity, as opposed to learning about it from others.
Beliefs
Deeply held convictions or opinions that influence an individual's actions and attitudes.
Q4: What is the hybridization of the I
Q9: Specify the hybridization of the nitrogen atom
Q14: _ phosphorus has a regular crystalline structure,
Q19: Four electrons are confined to a one-dimensional
Q40: Draw the Lewis structures of the molecules
Q42: Which of the following is not the
Q58: How many unshared pairs of electrons are
Q61: In the hydrogen spectrum, what is the
Q83: For the reaction A + B <font
Q88: Specify the number of unpaired electrons in