For the Reaction
CH3CHCH2(g) + HCl(g) CH3CHClCH3(g)
a Possible Mechanism Is Derive the Rate Law
For the reaction
CH3CHCH2(g) + HCl(g) CH3CHClCH3(g)
a possible mechanism is 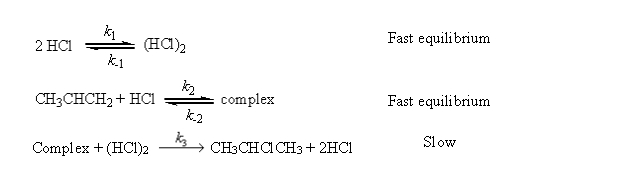 Derive the rate law for this reaction using this mechanism.
Derive the rate law for this reaction using this mechanism.
Definitions:
Q7: <font face="symbol"></font>S for this process is<br>A) greater
Q10: Which ion is planar?<br>A) CO<sub>3</sub><sup>2</sup><sup>-</sup><br>B) SO<sub>4</sub><sup>2</sup><sup>-</sup><br>C) PCl<sub>4</sub><sup>+</sup><br>D)
Q42: Choose the element with the smallest ionization
Q46: A metal crystallizes in a body-centered unit
Q48: A solution of two liquids, A and
Q50: Which of the following compounds has the
Q79: The normal boiling point of liquid X
Q83: Consider the pseudo-octahedral complex of Cr<sup>3+</sup> shown
Q100: Choose the correct molecular structure for PBr<sub>5.</sub><br>A)
Q108: The hypothetical reaction<br>2A <span class="ql-formula"