Multiple Choice
Complete the Lewis structure for the following molecule. 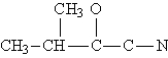 This molecule has __________ sigma bonds and __________ pi bonds.
This molecule has __________ sigma bonds and __________ pi bonds.
Distinguish between different types of cognitive abilities and their relation to gender.
Understand the controversies surrounding intelligence and cognitive ability research.
Analyze and critique the evidence for and against gender differences in specific cognitive tasks and areas.
Identify the factors that determine social class in Canada.
Definitions:
Related Questions
Q16: Fe<sup>2+</sup> + 2e<sup>-</sup> <span class="ql-formula"
Q20: The fact that O<sub>2</sub> is paramagnetic can
Q23: SO<sub>2</sub><br>A)Give the shape of the molecule.<br>B)Indicate the
Q33: Choose the group containing the most reactive
Q39: Lithium metal with Br<sub>2</sub>(l)
Q42: In the balanced cell reaction, what is
Q43: Choose the metal that is produced by
Q57: Which of the following would be the
Q113: What is the wavelength of light that
Q117: For an electron in a 2.00-nm one-dimensional