The energy expressions for the electrons in the He+ ion and the hydrogen atom are En (H) = -a/n2 and En (He+) = -4a/n2
Which of the following statements is(are) correct?
I. For the transitions 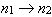 , the frequency is larger for H than for He+.
, the frequency is larger for H than for He+.
II. The first ionization energy of the H atom is smaller than the second.
III. The 1s orbital in He+ is larger (in the sense that the probability density is
shifted outward) than the 1s orbital in H.
Definitions:
Digestive System
Organ system that consists of digestive organs (stomach, intestines, etc.) and accessory organs (liver, etc.); ingests and digests food; absorbs nutrients and eliminates wastes.
Photosynthesis
A biochemical process in green plants and certain other organisms that converts light energy into chemical energy, using carbon dioxide and water to produce glucose and oxygen.
By-product
A secondary product generated as a result of a manufacturing process, chemical reaction, or biological pathway, which was not the primary product or goal.
Thermodynamics
The branch of physics that deals with the relationships between heat and other forms of energy, and how energy affects matter.
Q7: Bernie Nohave is operating a scheme whereby
Q7: A concentration cell is constructed using two
Q12: Which of the following statements is incorrect?<br>A)
Q22: Ross's family lives in Ontario. His parents
Q30: Elemental magnesium crystallizes in a face-centered cubic
Q38: The Queen's Wardrobe, a dressmaking boutique, agrees
Q80: Which molecule or ion violates the octet
Q92: When some Cl<sub>2</sub>(g) is added at constant
Q95: What does X represent in the Lewis
Q101: What is q?<br>A) greater than zero<br>B) More