The energy equation for a particle in a cubic box of dimensions Lx = Ly = Lz is
Enx, ny, nz = 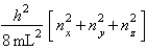 Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
A)In the ground state, how many of the 8 electrons have energy equal to 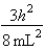 ?
?
B)In the ground state, how many of the 8 electrons have energy equal to 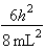 ?
?
C)In the ground state, how many of the 8 electrons have energy equal to 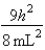 ?
?
D)Calculate the wavelength of light necessary to promote the highest-energy ground-state electron into the lowest-energy excited state. Assume a cubic box with dimensions 1.50 nm * 1.50 nm * 1.50 nm.
Definitions:
Notice
Formal information or warning given to a person or public about an event, decision, or action.
Assignment Valid
Refers to the legal transfer of one party's rights and benefits under a contract to another party, recognized as legitimate and binding.
Delegable
Capable of being assigned or entrusted to another person or party, particularly in the context of duties or authority.
Contractual Duty
An obligation imposed by a contract that requires a party to act or refrain from acting in a certain manner.
Q5: Most professionals are members of professional associations
Q26: What is the electron configuration for Cr<sup>2+</sup>?<br>A)
Q38: The Queen's Wardrobe, a dressmaking boutique, agrees
Q57: Which of the following would be the
Q58: What is the hybridization of the B
Q73: Select the correct molecular structure for SF<sub>5</sub><sup>+</sup>.<br>A)
Q78: In which of the following cases must
Q103: An electron in a 10.0-nm one-dimensional box
Q113: What is the shape of the ICl<sub>5</sub>
Q117: <font face="symbol"></font>S<sub>surr</sub> for this process is<br>A) less