The energy expressions for the electrons in the He+ ion and the hydrogen atom are En (H) = -a/n2 and En (He+) = -4a/n2
Which of the following statements is(are) correct?
I. For the transitions 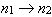 , the frequency is larger for H than for He+.
, the frequency is larger for H than for He+.
II. The first ionization energy of the H atom is smaller than the second.
III. The 1s orbital in He+ is larger (in the sense that the probability density is
shifted outward) than the 1s orbital in H.
Definitions:
Proprietor
The owner of a business or property.
Liabilities
Financial obligations or debts owed by a person or company to another entity, which must be settled over time.
Franchise Rule
is a regulation designed to provide prospective franchisees with the necessary information to make informed decisions about their investments in franchise operations.
Potential Earnings Figures
Estimates or projections of the amount of money that could be earned in the future, often used in the context of investments or employment.
Q17: Sodium oxide (Na<sub>2</sub>O) crystallizes in a structure
Q18: A plot of k vs. 1/T gives
Q54: A Canadian multinational was interested in developing
Q56: [Kr] 4d<sup>10</sup>5s<sup>2</sup>5p<sup>1</sup>
Q77: In the Lewis structure for elemental nitrogen,
Q81: [Ar] 4s<sup>2</sup>3d<sup>7</sup>
Q85: Which letter shows the activation energy?
Q92: MnO has either a structure like NaCl
Q104: For the reaction<br>P<sub>4</sub>(g) + 5O<sub>2</sub>(g)
Q136: What is <font face="symbol"></font>G at 25°C for