The energy equation for a particle in a cubic box of dimensions Lx = Ly = Lz is
Enx, ny, nz = 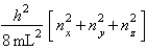 Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
A)In the ground state, how many of the 8 electrons have energy equal to 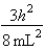 ?
?
B)In the ground state, how many of the 8 electrons have energy equal to 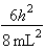 ?
?
C)In the ground state, how many of the 8 electrons have energy equal to 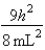 ?
?
D)Calculate the wavelength of light necessary to promote the highest-energy ground-state electron into the lowest-energy excited state. Assume a cubic box with dimensions 1.50 nm * 1.50 nm * 1.50 nm.
Definitions:
Nezelof's Syndrome
A rare immunodeficiency disorder characterized by a decreased function of the thymus gland, leading to low immunity and vulnerability to infections.
Idiopathic Aplastic Anemia
A rare, life-threatening disorder where the bone marrow fails to produce sufficient new cells to replenish blood cells, with unknown cause.
Weakness
A lack of strength or energy in the body or a specific part of the body, making physical activities more difficult.
Mediterranean Anemia
Another name for Thalassemia, a genetic blood disorder characterized by less oxygen in the body due to a decrease in hemoglobin.
Q4: The half-life is constant.<br>A) zero order in
Q5: The rate constant for a reaction increases
Q9: Rate constants are dependent upon <br>A) the
Q24: For the elements Rb, F, and O,
Q53: What is the electron configuration of Ti<sup>2+</sup>?<br>A)
Q66: Calculate E at 25°C for this cell,
Q82: The heat of combustion of bituminous coal
Q90: For the reaction 2N<sub>2</sub>O<sub>5</sub>(g) <font face="symbol"></font> 4NO<sub>2</sub>(g)
Q95: Which of the following atoms has 3
Q100: When a metal and a nonmetal chemically