Which metal, Al or Ni, could reduce Zn2+ to Zn(s) if placed in a Zn2+(aq) solution? 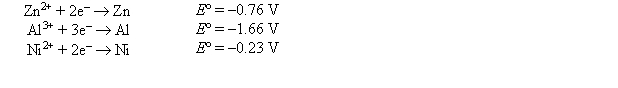
Definitions:
Usage Rate
The frequency at which a product is used by the consumer.
Subscription Basis
A payment model in which customers are charged a recurring fee at regular intervals for access to a product or service.
Multiple Treatments
The application or use of several therapeutic interventions or methods simultaneously or in a series to address a particular condition or issue.
Usage Rate
The frequency at which a consumer uses a product or service, often influencing marketing strategies and product development.
Q10: Nancy was the administrator of her deceased
Q17: Terry responded to a mail-order advertisement for
Q21: What form will the pseudo-rate law have?<br>A)
Q38: On a cold winter day, A slipped
Q42: A quantum chemistry program indicates that the
Q45: Given the following free energies of formation:
Q49: James, a 16-year-old boy, got a job
Q53: Which of the following statements about the
Q65: Determine which of the following atoms is(are)
Q94: Calculate <font face="symbol"></font>S for cooling 1.9 mol