Which of the following result(s) in an increase in the entropy of the system? 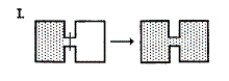 I. (See diagram.) II. Br2(g) Br2(l)
I. (See diagram.) II. Br2(g) Br2(l)
III. NaBr(s) Na+(aq) + Br-(aq)
IV. O2(298 K) O2(373 K)
V. NH3(1 atm, 298 K) NH3(3 atm, 298 K)
Definitions:
Distractions
Things that divert an individual's attention from the desired point of focus, potentially reducing productivity or performance.
Free-Form Designs
Creative layouts or structures that are not restricted by traditional shapes or patterns.
Dynamic Experience
An interactive or engaging user experience that adapts based on user actions or preferences.
Online Presentations
Digital visual materials prepared and displayed over the Internet to convey information or ideas to an audience remotely.
Q1: Which element is most likely to form
Q3: Smith, a computer programmer with Computer Company,
Q5: The rate constant for a reaction increases
Q7: <font face="symbol"></font>S for this process is<br>A) greater
Q16: How many of the atoms are sp<sup>2</sup>
Q18: Cu
Q57: Which of the following would be the
Q66: Calculate E at 25°C for this cell,
Q100: What is the value of
Q129: In which pair do both compounds exhibit