Which of the following result(s) in an increase in the entropy of the system? 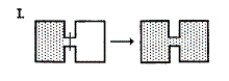 I. (See diagram.) II. Br2(g) Br2(l)
I. (See diagram.) II. Br2(g) Br2(l)
III. NaBr(s) Na+(aq) + Br-(aq)
IV. O2(298 K) O2(373 K)
V. NH3(1 atm, 298 K) NH3(3 atm, 298 K)
Definitions:
Activity Levels
The volume of production or the level of sales activity within a company, which can affect various costs and pricing strategies.
High-low Method
An accounting technique used to estimate variable and fixed costs by analyzing the lowest and highest levels of activity and their associated costs.
Mixed Cost
An expense that contains both fixed and variable components, changing in total with the level of activity but not in a directly proportional manner.
High-low Method
A method employed in accounting that calculates variable and fixed expenses by analyzing the maximum and minimum activity levels.
Q2: Save-A-Lot Food Stores Limited regularly purchased large
Q12: How many electrons can be described by
Q19: Do directors and officers have liability for
Q26: Under environmental legislation, enforcement officers<br>A) are not
Q39: One afternoon you find a notebook filled
Q66: Select the correct molecular structure for IF<sub>6</sub><sup>+</sup>.<br>A)
Q75: Which element has the most unpaired electrons
Q93: The statement that the first ionization energy
Q107: In a certain reversible expansion, a system
Q114: One mole of an ideal gas is