Consider the following hypothetical reaction (at 308 K) . Standard free energies, in kJ/mol, are given in parentheses. 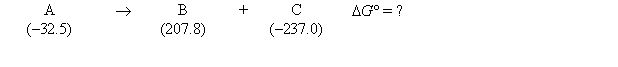 What is the value of the equilibrium constant for the reaction at 308 K?
What is the value of the equilibrium constant for the reaction at 308 K?
Definitions:
Future Cash Flows
Estimated amounts of money expected to be received or paid out by an entity in the future as a result of financial transactions or business operations.
Net Book Value
The value at which an asset is carried on a balance sheet, calculated as the asset's original cost minus accumulated depreciation and impairments.
Accelerated Depreciation
A method of depreciation where assets lose value at a faster rate in the initial years of their life.
Straight-Line Depreciation
A method of calculating the depreciation of an asset that allocates an equal amount of depreciation each year over the asset's useful life.
Q16: A and B entered a variety store
Q18: Anderson, a city employee, collected garbage along
Q28: A Canadian firm with a highly desirable
Q30: As a federal law, the Competition Act
Q31: Given the following information, determine the standard
Q42: From the following list of observations, choose
Q53: On a cold winter day, A slipped
Q97: What is the electron configuration of the
Q113: What is the shape of the ICl<sub>5</sub>
Q126: What is w?<br>A) greater than zero<br>B) More