A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C, and the pressure is increased to 8 atm. Based on the phase diagram below, what will happen? 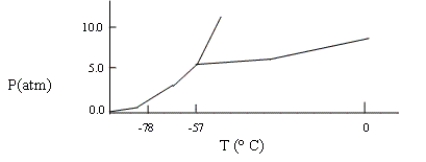
Definitions:
Machining
The process of removing material from a workpiece using power-driven machine tools to shape it into a desired form.
Activity-based Costing
Activity-based costing involves identifying and assigning costs to overhead activities and then assigning those costs to products or services based on their consumption of those activities, offering detailed insights into cost drivers.
Floral Bouquets
Arrangements of flowers often used for decorative purposes or given as gifts for special occasions, composed of various types of flowers and foliage.
Activity-based Costing
A costing method that assigns overhead and indirect costs to specific activities, improving the accuracy of product and service pricing.
Q7: What is the rate law for the
Q18: Radioactive elements decay via first-order kinetics. Consider
Q31: If more than one neutron from each
Q35: The bond order in the NO molecule
Q36: For which of the following diatomic molecules
Q53: How many unpaired electrons are found in
Q70: Consider an ionic compound C<sub>x</sub>A<sub>y</sub> where the
Q76: You have at your disposal 3 1-pound
Q114: How many of the following electron configurations
Q124: The distance at which the sum of