A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C, and the pressure is increased to 8 atm. Based on the phase diagram below, what will happen? 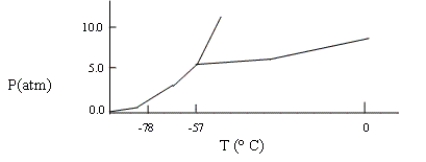
Definitions:
Behavior
The observable actions or responses of an organism, typically in response to its environment or internal cues.
Functionalism
James’s approach to mental processes, emphasizing the functions and purposes of the mind and behavior in the individual’s adaptation to the environment.
William James
An American philosopher and psychologist, often referred to as the father of American psychology.
Psychology
The scientific study of the mind and behavior, encompassing various aspects of conscious and unconscious experience as well as thought processes.
Q8: The number of orbitals having a given
Q10: Which of the following compounds contains only
Q16: What is the hybridization of the carbon
Q22: At a given temperature, the vapor pressure
Q29: What compounds are useful in breathing apparatus
Q30: Which of the following combinations of quantum
Q34: When 1 mol of a nonvolatile solvent-nondissociating
Q61: The phenomenon called the _ contraction is
Q113: _ is defined as the number of
Q123: What are the angles of the Se-F