Based on the phase diagram shown below, which of the following statements are correct?
I. Sublimation occurs at a point in the transformation that falls along a straight line from point A to point F.
II. C and E represent points where the gas and liquid phases are in equilibrium.
III. ΔHvap can be measured at point B.
IV. Molecules at point D have a greater average kinetic energy than those at point F.
V. The temperature at point E is called the critical temperature of the compound. 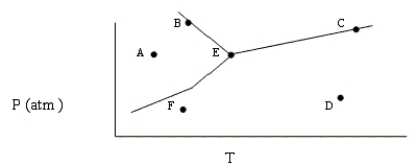
Definitions:
100% Bar Charts
Graphical representations where the total value of each bar equals 100% to show comparative ratios of different categories.
Organizational Charts
Diagrams that depict the structure of an organization, showing the relationships and relative ranks of its parts and positions/jobs.
Word-processing Software
Computer programs designed for creating, editing, formatting, and sometimes printing text-based documents.
Superscripts
Small characters or symbols set slightly above the normal line of text, often used in mathematical expressions, footnotes, or chemical formulae.
Q3: Which of the following statements is correct?<br>A)
Q15: What is the net number of face-centered
Q32: For the elements Rb, F, and O,
Q39: In which of the compounds below is
Q49: The reaction of hydrazine with O<sub>2</sub>(g)
Q61: The hypothetical reaction<br>2A → 2B + D<br>is
Q76: The reaction of chlorine gas with cold
Q95: Which of the following series is isoelectronic?<br>A)
Q97: Consider the following molecules.<br>I. BF<sub>3</sub><br>II. CHBr<sub>3</sub> (C
Q124: In general, Z is less than Z<sub>eff</sub>.