The reaction of (CH3) 3CBr with hydroxide ion proceeds with the formation of (CH3) 3COH.(CH3) 3CBr (aq) + OH- (aq) → (CH3) 3COH (aq) + Br- (aq)
The following data were obtained at 55°C. 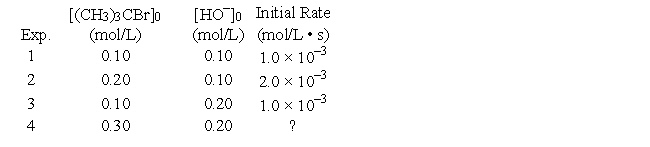
What will the initial rate (in mol/L • s) be in Experiment 4?
Definitions:
AVC
Average Variable Cost; the total variable costs divided by the number of units produced, indicating the variable expense per unit of output.
MP
Marginal Product, which refers to the increase in output that results from employing an additional unit of input, holding all other inputs constant.
Average Fixed Cost
Average Fixed Cost refers to the total fixed costs (costs that do not change with the level of output) divided by the quantity of output produced. It decreases as production increases.
Total Variable Cost
The total of expenses that vary directly with the level of production, such as raw materials and direct labor.
Q1: Which of the following has a central
Q7: What is the rate law for the
Q21: For how many of the following does
Q30: hexane (C<sub>6</sub>H<sub>14</sub>) and chloroform (CHCl<sub>3</sub>)<br>A) relatively ideal<br>B)
Q37: A chemist is given a white solid
Q51: Consider the following sets of quantum numbers.
Q59: Of the following homonuclear diatomic molecules, which
Q76: You have at your disposal 3 1-pound
Q80: Identify the major attractive force in HF.<br>A)
Q101: Of the three cubic unit cells, which