The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assume the form of the rate law is
Rate 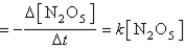 where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.
-What is the half-life for the reaction described?
Definitions:
Nonprogrammed
relates to decision-making or solutions that are not based on established procedures or guidelines, often required in unique, unforeseen, or complex situations.
Complex Nature
The intricate and multifaceted characteristics of a system, problem, or entity that make it challenging to understand or manage.
Appliance Safety Features
Refers to the built-in safety mechanisms and designs included in appliances to prevent accidents and injuries.
Kitchen Injuries
Injuries occurring in the kitchen, often involving cuts, burns, or slips due to handling knives, hot surfaces, or wet floors.
Q14: Which statement about N<sub>2</sub> is false?<br>A) It
Q22: Choose the correct molecular structure for NO<sub>3</sub><sup>-</sup>.<br>A)
Q32: For an electron in a one-dimensional box,
Q46: Which of the following ions interfere(s) with
Q52: If oxidation of H<sub>2</sub>O occurs at the
Q62: The reaction 2H<sub>2</sub>O<sub>2</sub> → 2H<sub>2</sub>O + O<sub>2</sub>
Q69: Identify the major attractive force in H<sub>2</sub>S.<br>A)
Q78: What is the hybridization of the central
Q98: The valence electron configuration of an element
Q99: In which orbital does an electron experience