The reaction 2NO → N2 + O2 has the following rate law: 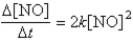 After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
Definitions:
Individual Contribution
The unique input or effort one person provides towards a group task or project.
Pluralistic Ignorance
A social psychological phenomenon where a majority of group members privately reject a norm, but incorrectly assume that most others accept it, and therefore go along with it.
Psychology Class
An academic course that studies the mind and behavior, covering topics from social interactions to cognitive processes.
Stereotype
A widely held but oversimplified and generalized belief about a particular type of person, group, or thing, which may or may not reflect reality.
Q8: Which of the following statements is/are true
Q9: The configuration (σ<sub>2s</sub>)<sup>2</sup>(σ<sub>2s</sub>*)<sup>2</sup>(π<sub>2py</sub>)<sup>1</sup>(π<sub>2px</sub>)<sup>1</sup> is the molecular orbital
Q13: How many of the atoms are sp
Q14: At a given temperature, you have a
Q47: You are given a small bar of
Q55: Which of the following statements is/are true
Q61: [Ar] 4s<sup>2</sup>3d<sup>7</sup>
Q73: The ion that aluminum is most likely
Q93: Choose the metal with the lowest melting
Q105: Identify the order that is observed experimentally