The following questions refer to the reaction 2A2 + B2 → 2C. The mechanism below has been proposed: 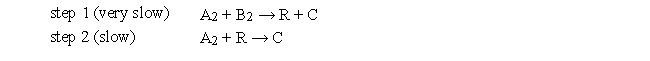
-When ethyl chloride, CH3CH2Cl, is dissolved in 1.0 M NaOH, it is converted into ethanol, CH3CH2OH, by the reaction
CH3CH2Cl + OH- → CH3CH2OH + Cl-
At 25°C the reaction is first order in CH3CH2Cl, and the rate constant is 1.0 × 10-3 s-1. If the activation parameters are A = 3.4 × 1014 s-1 and Ea = 100.0 kJ/mol, what will the rate constant be at 36°C? (R = 8.314 J/mol • K)
Definitions:
Mutual Fund Prospectus
A legal document provided by mutual funds to potential investors, detailing the fund's objectives, risks, performance, and expenses.
Front-end Loads
Charges paid by investors at the time of purchasing a mutual fund.
Back-end Loads
Fees paid by investors when selling mutual fund shares, typically decreasing over time the longer the shares are held.
Hedge Fund
A private investment fund that employs a variety of strategies to earn active return, or alpha, for their investors, often involving leverage, derivatives, and short selling.
Q2: A concentration cell is constructed using two
Q3: Which of the following chemical or physical
Q41: What is the electron arrangement around the
Q45: Choose the molecule with the strongest bond.<br>A)
Q70: How many unpaired electrons are found in
Q80: How many electrons can be described by
Q82: Alkali halides commonly have either the sodium
Q85: Choose the only element that forms a
Q91: Chromium metal crystallizes as a body-centered cubic
Q99: Select the correct molecular structure for XeCl<sub>4</sub>.<br>A)